Mercury Cycling in the Environment
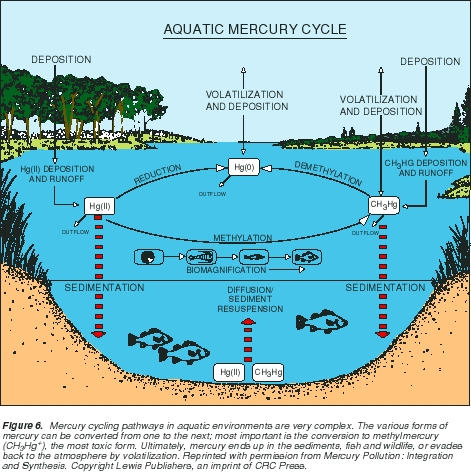
Mercury cycling pathways in aquatic environments are very complex. The various forms of mercury can be converted from one to the next; most important is the conversion to methylmercury (CH3Hg+), the most toxic form. Ultimately, mercury ends up in the sediments, fish and wildlife, or evades back to the atmosphere by volatilization.
 Aquatic Mercury Cycle Aquatic Mercury Cycle
At right, Figure 6 shows a schematic drawing of mercury cycling in an aquatic ecosystem.1 With the exception of isolated cases of known point sources, the ultimate source of mercury to most aquatic ecosystems is deposition from the atmosphere, primarily associated with rainfall.
As depicted in this figure, atmospheric deposition contains the three principal forms of mercury, although the majority is as inorganic mercury (Hg2+, ionic mercury). Once in surface water, mercury enters a complex cycle in which one form can be converted to another. It can be brought to the sediments by particle settling and then later released by diffusion or resuspension. It can enter the food chain, or it can be released back to the atmosphere by volatilization.
The concentration of dissolved organic carbon (DOC) and pH have a strong effect on the ultimate fate of mercury in an ecosystem. Studies have shown that for the same species of fish taken from the same region, increasing the acidity of the water (decreasing pH) and/or the DOC content generally results in higher body burdens in fish. Many scientists currently think that higher acidity and DOC levels enhance the mobility of mercury in the environment, thus making it more likely to enter the food chain.
Many of the details of the aquatic mercury cycle are still unknown, however, and remain areas of active research.
How does mercury enter the food chain?
The exact mechanism(s) by which mercury enters the food chain remain largely unknown, and probably vary among ecosystems. We do know, however, that certain bacteria play an important early role. Studies have shown that bacteria that process sulfate (SO4=) in the environment take up mercury in its inorganic form, and through metabolic processes convert it to methylmercury. The conversion of inorganic mercury to methylmercury is important for two reasons: (1) methylmercury is much more toxic than inorganic mercury, and (2) organisms require considerably longer to eliminate methylmercury. At this point, the methylmercury-containing bacteria may be consumed by the next higher level in the food chain, or the bacteria may release the methylmercury to the water where it can quickly adsorb to plankton, which are also consumed by the next level in the food chain.
Where does atmospheric mercury come from?
There are many sources of mercury to the environment, both natural and man related. Natural sources include volcanoes, natural mercury deposits, and volatilization from the ocean. The primary human-related sources include: coal combustion, chlorine alkali processing, waste incineration, and metal processing. Best estimates to date suggest that human activities have about doubled or tripled the amount of mercury in the atmosphere, and the atmospheric burden is increasing by about 1.5 percent per year.
Has there always been mercury contamination, or is this a recent problem?
This is a difficult question to answer, in part because of a lack of adequately preserved fish specimens of preindustrial age to compare against contemporary samples. However, several lines of evidence from recent studies on Wisconsin lakes suggest that increase pemissions to the atmosphere, and subsequent higher deposition rates to lakes, likely translate into higher mercury levels in fish. Although the total amount of mercury delivered to one of these lakes annually is very small it is strongly absorbed by organic material floating in the water such as plankton or bacteria. These microorganisms are consumed by organisms higher in the food chain, or after dying, settle to the bottom of the lake and are incorporated into bottom sediments. Studies of sediment cores show that younger sediments deposited since industrialization have mercury concentrations that are about 3-5 times that of historical sediments. Thus, the fact that these sediments are primarily composed of dead microorganisms that were once the bottom of the food chain would suggest that modern levels of mercury in the food chain are elevated over preindustrial times.
If human-related emissions could be eliminated or reduced, how long would it take for ecosystems to recover?
The only way to attempt to answer this question is to incorporate all the best information currently available on how mercury behaves in the environment into a computer model. Such a model was constructed as part of the research effort on northern Wisconsin lakes. Modeled scenarios predict that if emissions could be reduced by 5 percent, it would take 8 years before any change in fish concentrations would be observed, and the decrease would be small.

Read a more complete explanation of the mercury cycle:
USFS Fact Sheet FS 216-95, Mercury Contamination of Aquatic Ecosystems, by Dave Krabbenhoft and David Rickert (1995).
References
1) Figure 6 reprinted with permission from Mercury Pollution: Integration and Synthesis. Copyright Lewis Publishers, an imprint of CRC Press.
|

